Oak family (Fagaceae)
Oaks (Quercus spp.), chestnut (Castanea sp.), and American beech (Fagus grandifolia)

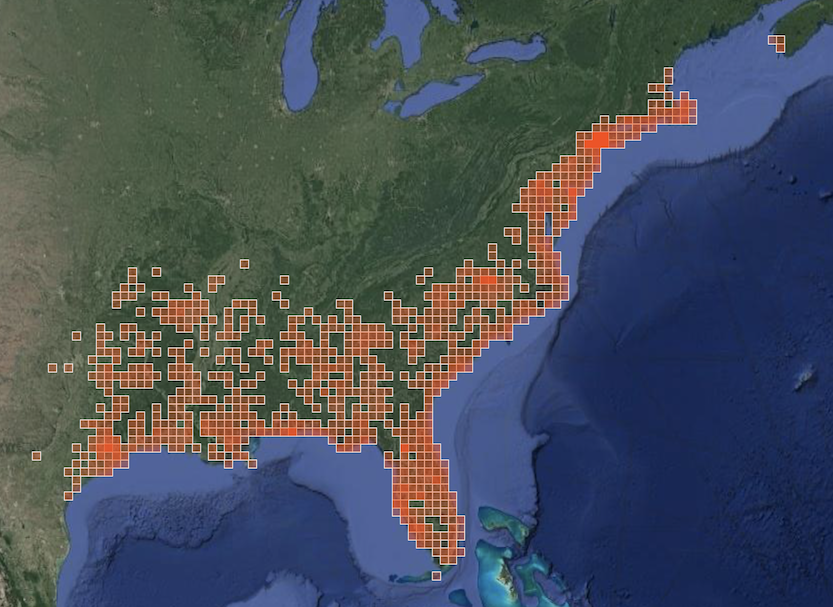
Fagaceae specialists presumed to breed locally, mainly on chestnuts [#records, 2014–2022 unless otherwise noted]
Tischeria quercitella Clemens, 1863 [4 records, all Nathan Mott Park; known hosts of this species are oak and chestnut]
Coptotriche citrinipennella (Clemens, 1859) [10 adult records, of which 6 in Nathan Mott Park, 2 at the Scout camp; also reared from chestnut sapling in Nathan Mott Park and photographed once at Grindley property; known hosts of this species are oak and chestnut]
Phyllonorycter kearfottella (Braun, 1908) [4 records, all single location in Nathan Mott Park on three different nights; chestnut is the only known host of this species]
Neurobathra strigifinitella (Clemens, 1860) [25 records in 2018–2021 quantitative data, of which 14 in Nathan Mott Park, 5 at the Scout camp, 4 at Hunt property; photographed once at Grindley property; known hosts of this species are oak, chestnut, and beech]
Coleotechnites quercivorella (Chambers, 1872) [several records, most or all in Nathan Mott Park; records need to be checked for completeness; oak is the only known host genus of this species, but Block Island records strongly suggest chestnut is a suitable host]
Pseudotelphusa quercinigracella (Chambers, 1872) [7 records, of which 6 in Nathan Mott Park and 1 at Hunt property; species may not be known from chestnut, but Block Island records strongly suggest it is a suitable host]
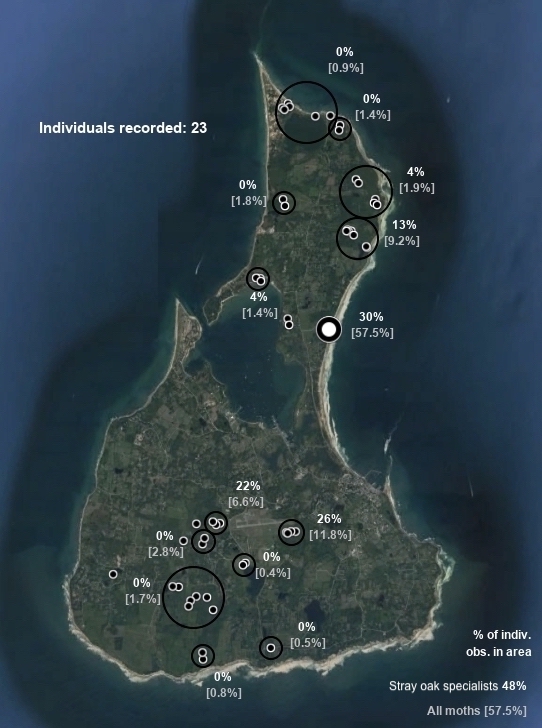
Nadata gibbosa (Smith, 1797) [25+ records; larvae prefer Fagaceae but have been found on other trees; on Block Island, most records are from Nathan Mott Park and the Scout camp, suggesting the population relies at least mainly on chestnut and beech; two larvae found on chestnut in Nathan Mott Park]
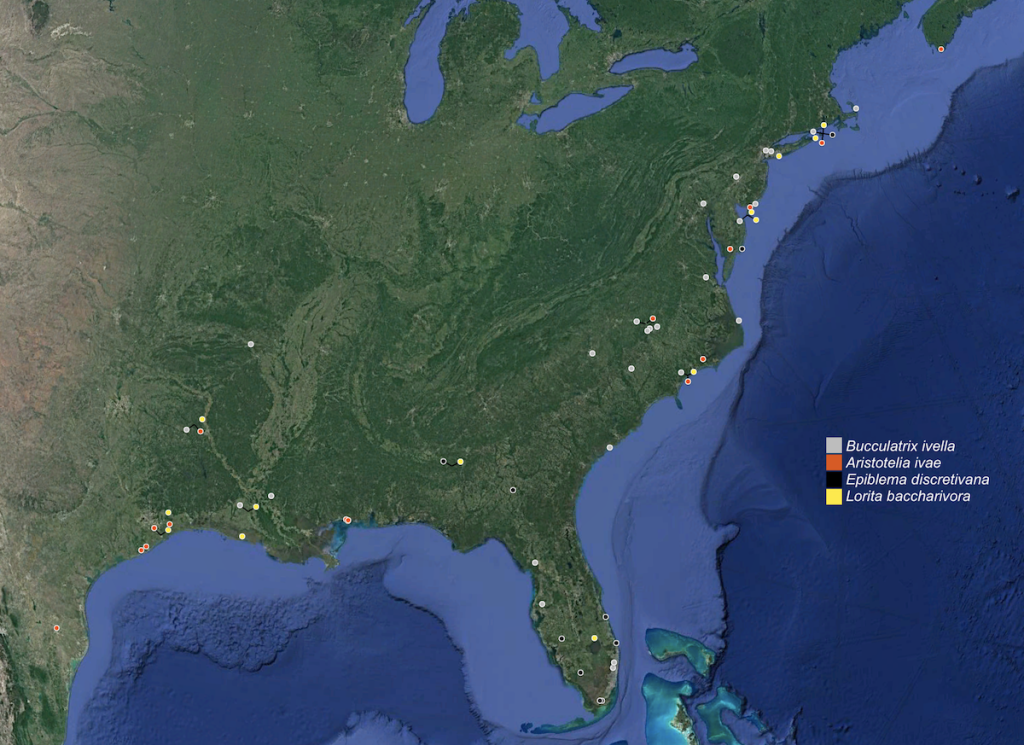
Fagaceae specialists recorded on Block Island likely only as strays [#records, 2014–2022]
Nepticulidae: Stigmella macrocarpae (Freeman, 1967) [1]
Bucculatricidae: Bucculatrix packardella Chambers, 1873 [1]
Gracillariidae: Phyllonorycter fitchella (Clemens, 1860) [1]
Gelechiidae: Trypanisma prudens Clemens, 1860 [1]; Pubitelphusa latifasciella (Chambers, 1875) [1]; Xenolechia ontariensis Keifer, 1933 [3]; Chionodes pereyra Clarke, 1947 [1]; Chionodes sevir Hodges, 1999 [2]; Chionodes thoraceochrella (Chambers, 1872) [3]
Tortricidae: Chimoptesis pennsylvaniana (Kearfott, 1907) [1]; Acleris semipurpurana (Kearfott, 1905) [2]; Argyrotaenia quercifoliana (Fitch, 1858) [2]; Archips semiferanus (Walker, 1863) [2]
Pyralidae: Oneida lunulalis (Hulst, 1887) [1]; Salebriaria turpidella (Ragonot, 1888) [1]; Salebriaria engeli (Dyar, 1906) [1]
Geometridae: Besma quercivoraria (Guenée, [1858]) [4]
Noctuoidea: Peridea angulosa (Smith, 1797) [1]; Macrurocampa marthesia (Cramer, 1780) [1]; Heterocampa obliqua Packard, 1864 [4 — all same night near two oaks in Scout camp, potentially offspring of stray gravid female]; Catocala dejecta Strecker, 1880 [1]; Catocala ilia (Cramer, 1775) [2]; Catocala connubialis Guenée, 1852 [1]; Catocala lineella Grote, 1872 [1]; Acronicta modica (Walker, 1856) [0 — unconfirmed, before 1996]; Acronicta lithospila (Grote, 1874) [1]; Cosmia calami (Harvey, 1876) [4]
Status unclear [#records, 2014–2022]
Blastobasis glandulella (Riley, 1871) [13 records, suggesting species may breed locally utilizing undocumented host; the species is abundant regionally]
Sereda tautana (Clemens, 1865) [only one record; probably a stray but possibly breeding on pair of oaks at Scout camp and otherwise missed due to early flight]
Pococera expandens (Walker, 1863) [several records on Block Island without geographic association with Fagaceae, and this is not an especially common species regionally; may have additional host]
Cyclophora packardi (Prout, 1936) [9 records: 2 at the Scout camp, 3 at Hunt property, 2 at Rohn property possibly misidentified C. myrtaria, 2 at Grindley property; surely breeds on island, likely utilizing host other than oak]
Hyperstrotia pervertens (Barnes & McDunnough, 1918) [4 records on Block Island without geographic association with Fagaceae; may have additional host]