About blockislandmoths.org
Block Island Moths is intended for use both by anyone looking learn about Block Island’s moth fauna and for professional and amateur lepidopterists more generally. This website can serve as a field guide for use by anyone attempting identification of moths found on Block Island, though it would be of more limited use elsewhere in the region, since there are many species commonly found on the mainland and on nearby islands that do not occur on Block Island. If you find moths of any life stage on Block Island, please submit photos to BugGuide or iNaturalist or send them to me at blockislander1@gmail.com for prompt identification and inclusion of any novel species or seasonality records here.
The main section of this website is for moths, with butterflies excluded. However, there are sections for the butterflies and caddisflies of Block Island. The species pages for butterflies lack the phenology graph and distribution map found on the moth species pages, since butterflies are all diurnal and seldom seen at lights. I have recorded caddisflies at lights using the same methodology as for moths, so species pages for Trichoptera include these graphics.
In addition to providing a wealth of information on Block Island’s moth fauna, this website serves as a digital field guide to the island’s moths. There may be no place on Earth with 1,000+ moth species where identification of the local fauna is as easy as it now is on Block Island thanks to this website. Go to the identify section to identify nearly any moth on Block Island using plates of live images.
The phenology data included on the species pages would probably prove instructive for regional comparisons and useful as a guide to species’ flight times to moth observers throughout southern New England. The data on species’ local distribution, combined with the information included on species’ habitat preferences and host plants, could also prove useful as a proxy measure of the distribution of Block Island’s flora. Finally, the summaries of the data gathered at each black lighting site can provide some indication of the most ecologically valuable places on Block Island especially worthy of conservation.
Identification
This section of the Block Island Moths website is intended to be used as a guide to the island’s moth fauna. It is obtimized for mobile browsers to enable easy use on a phone as a digital field guide. The images are organized multiple ways to aid identification.
The taxonomy view displays single images of up to six species in each superfamily, family, genus, or other taxon. In this view, taxa are organized and images selected with ease of use and aesthetics in mind; the ordering therefore differs substantially from both the Hodges (1983) sequence and the phylogenetic order of Pohl, Patterson, and Pelham (2016). Generally, similar taxa are placed together, and the most abundant and diverse taxa are placed near the top. Clicking on a taxon will bring the user to a page displaying images of all local daughter taxa. When a taxon has only one immediate daughter taxon present on Block Island, clicking on it skips to the daughter taxon. When viewing genera, for any genus with only one local species, the species name will be shown. Clicking on it will bring the user to its species page. Clicking on a genus displays an image of each species in the genus; displaying an image of each species for a higher taxon can be done by selecting either of the other two viewing options (see next). The species view displays a single image of each species in a given taxon. The default is for the species to be ordered according to the Pohl et al. (2016) phylogenetic sequence, but the species can instead be displayed in order of abundance in my surveying from 2018 to 2021.
Species Information
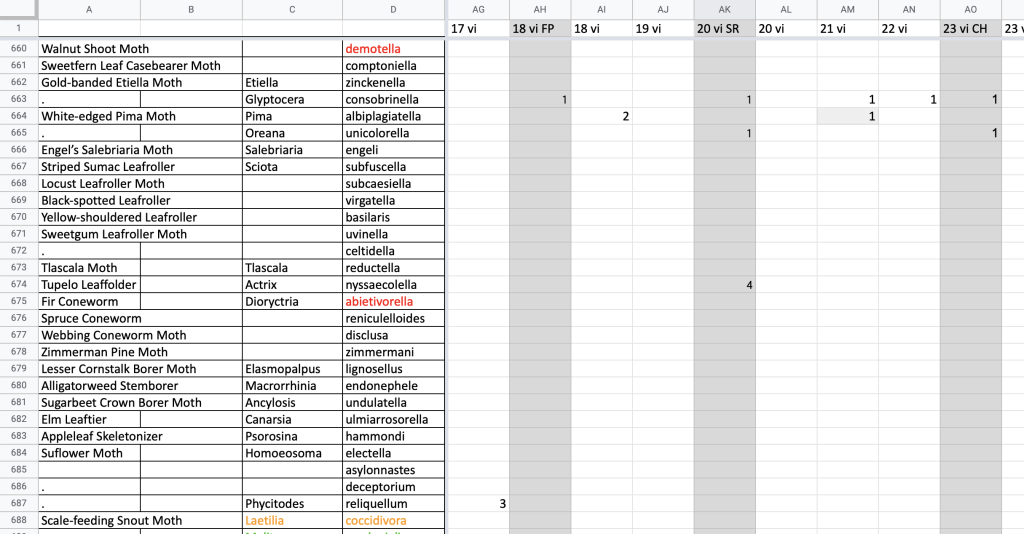
The centerpiece of this website is the series of species pages for the more than 1,200 moth species recorded on Block Island. Each page includes representative live photographs; a chart of flight time phenology; a map of Block Island depicting the local distribution of the species; information on host plants, life cycle, identification, and more; and a list of specimens collected on Block Island.
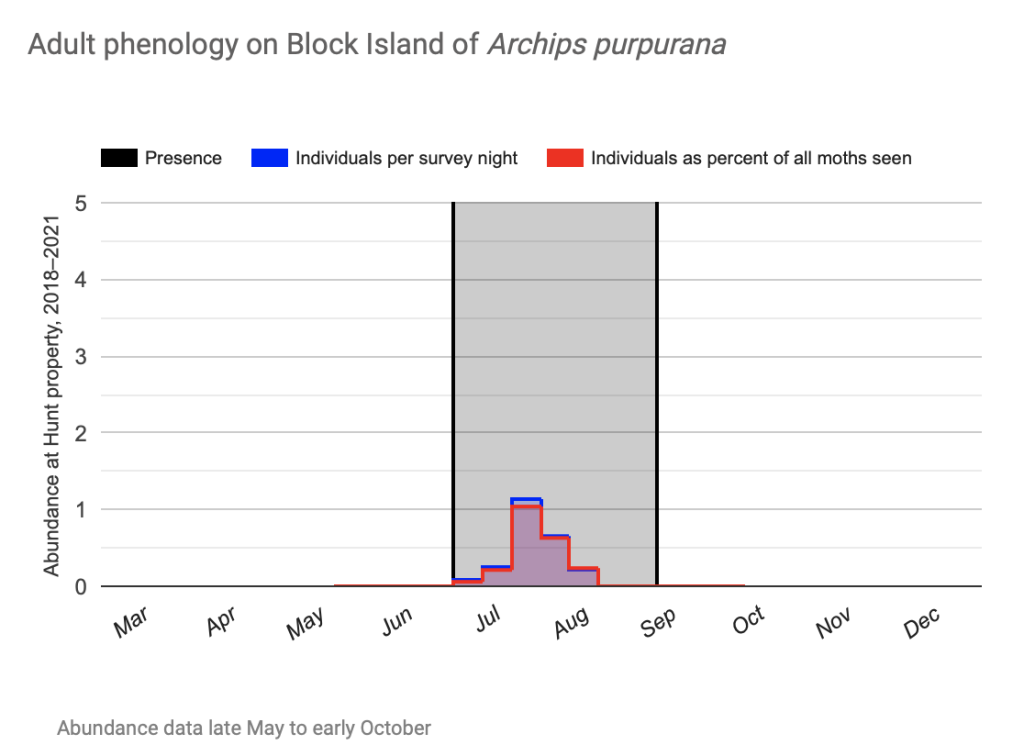
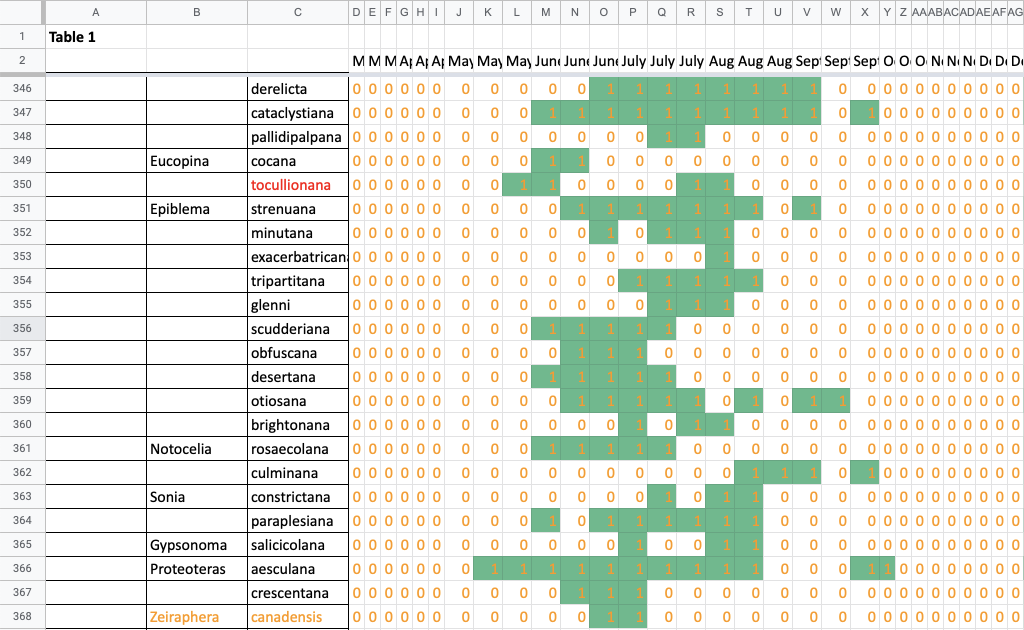
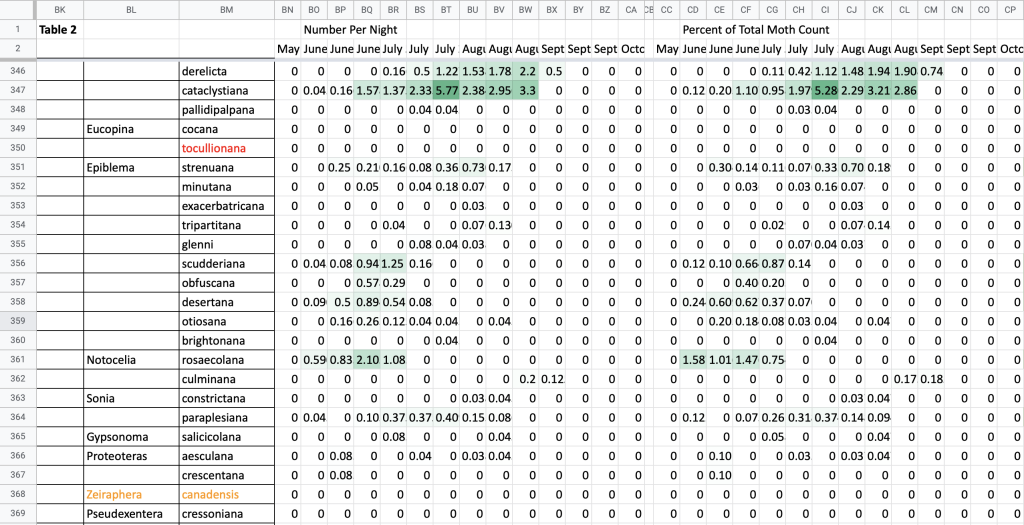
Flight time phenology is represented for most species in three ways, in all cases organized by 1/3-month period (1st to 10th, 11th to 20th, and 21st to end of month) (Fig. 1). All records from photographs, notes, and specimens gathered in the present survey, along with all older records I have found from previous surveying, are used as species presence records. To calculate abundance, I use only my nightly records from my porch lights (the Hunt property), which I began taking in 2018. I have enough records for abundance estimates from late May to early October. Abundance is represented in two ways: as individuals per survey night and as percent of moths of all species.
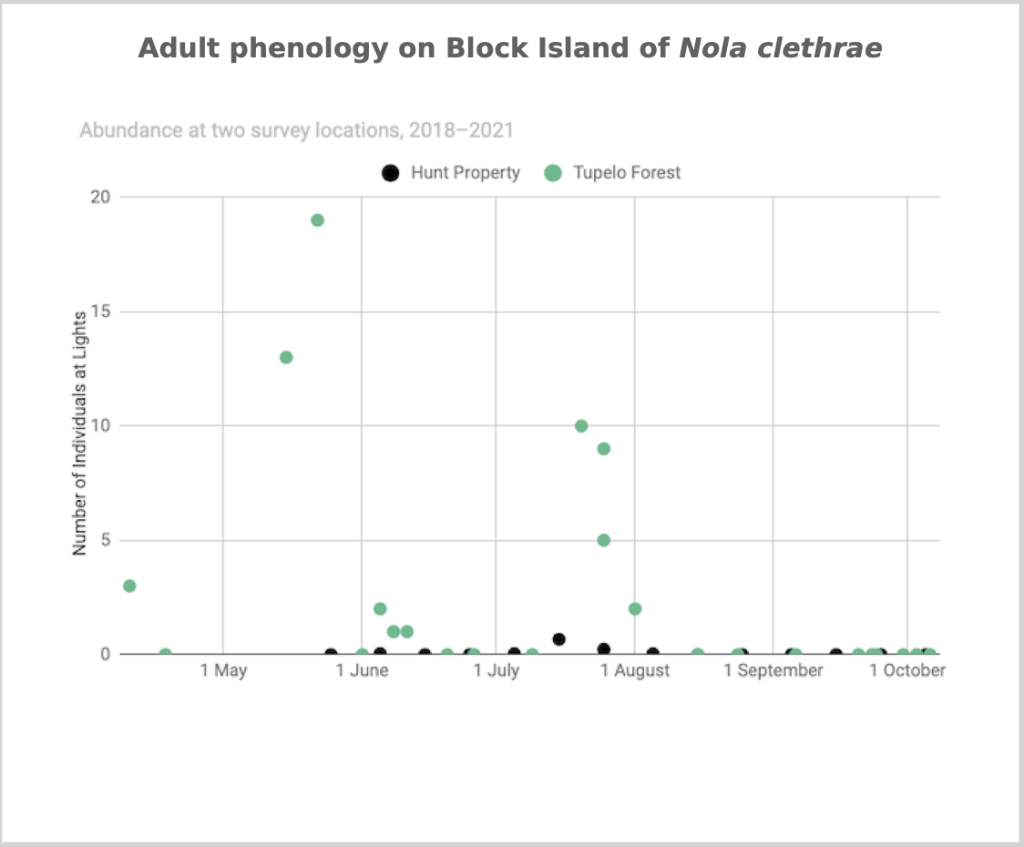
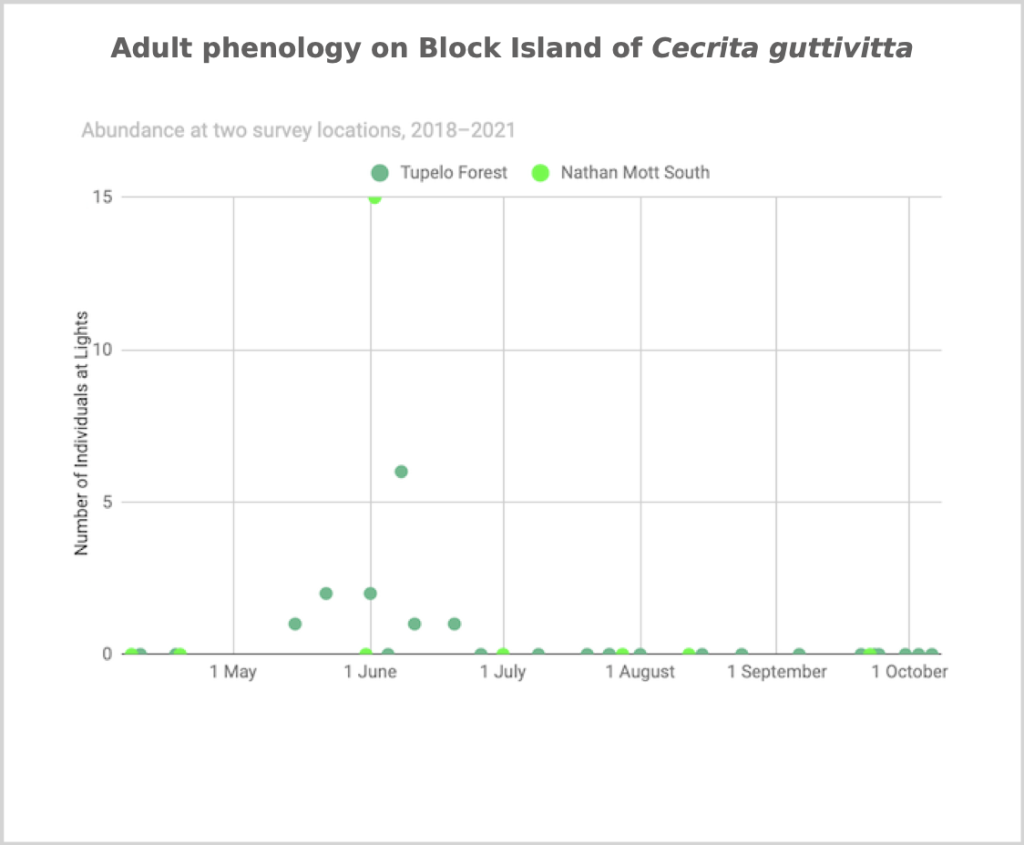
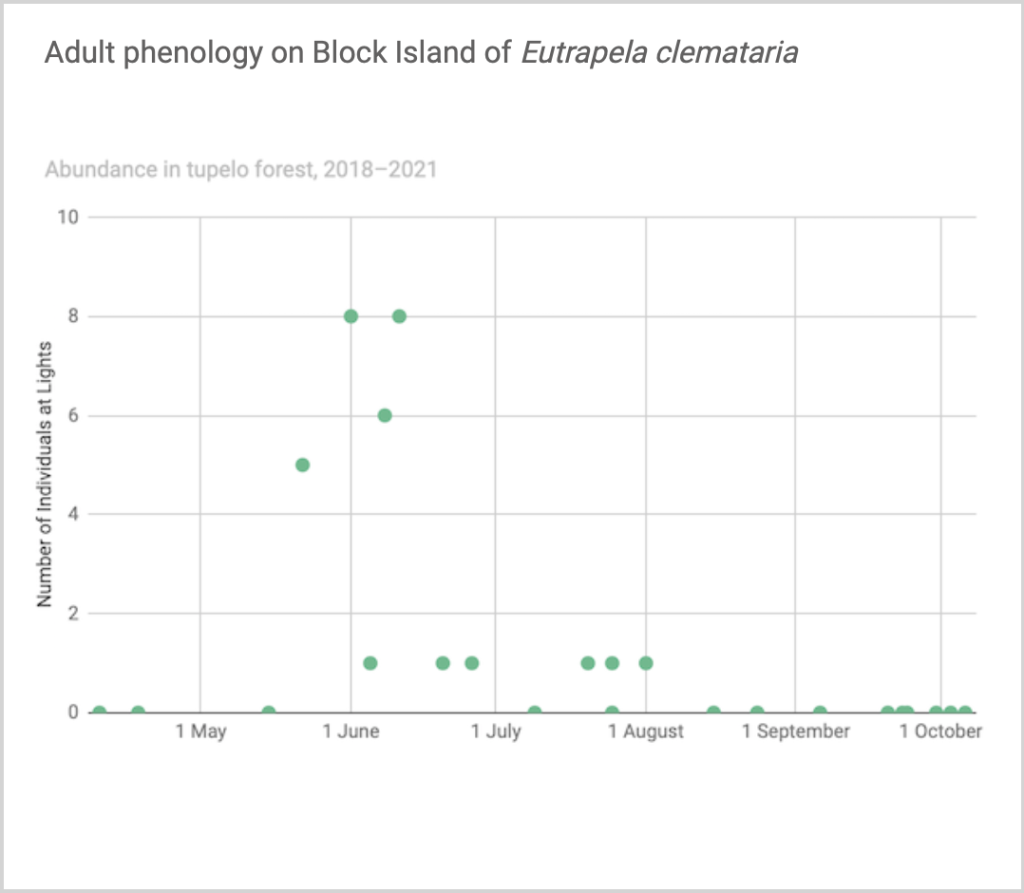
Flight time phenology is represented differently for species that are rare at the Hunt property but common enough at at least one major black lighting site to create a large sample size. More than half of all my records since 2018 are at the Hunt property, and this is the only site with many survey nights in each 1/3-month period throughout the summer. For species common at the tupelo forest southeast of the airport and/or the site in the north of Nathan Mott Park Preserve west of the airport and/or the site in the south of Nathan Mott Park Preserve near Old Mill Road but rare at the Hunt property, numbers of individuals for each survey night from one or both sites are depicted in a scatterplot, with abundance values for the Hunt property added if informative (Figs. 2–4).
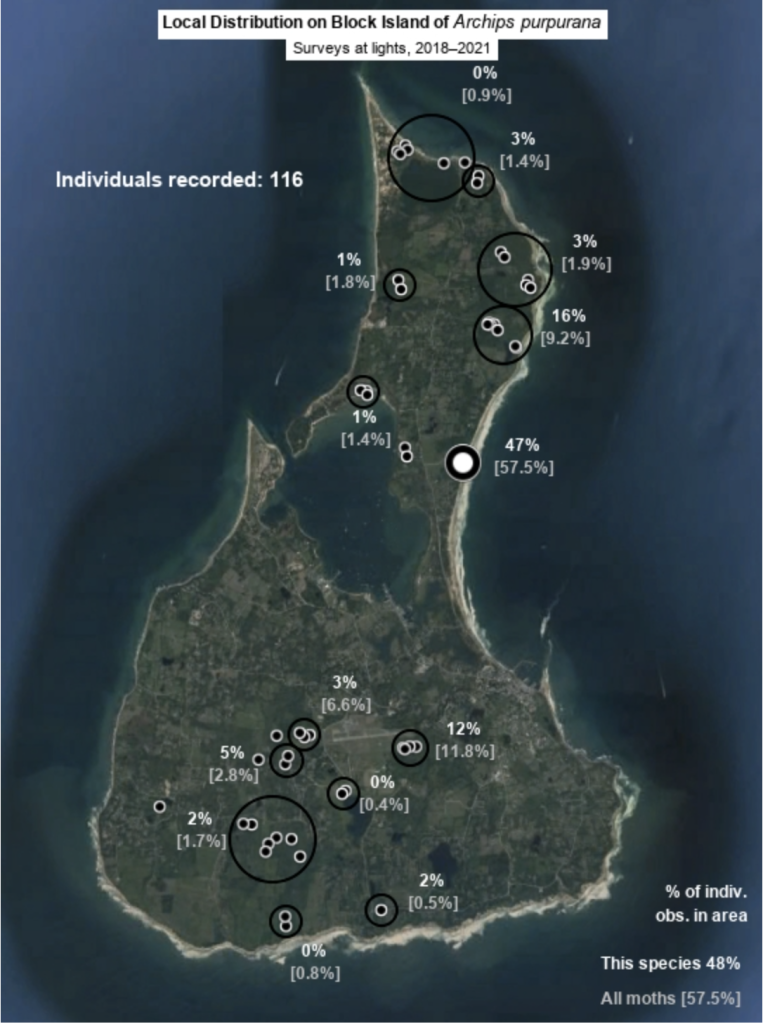
Each species page include a map of Block Island that provides information on the local distribution of the species (Fig. 5). Each map uses my sighting records from the Hunt property and at sheets at sites across Block Island since 2018. The total number of sightings of the species, the percentages of those sightings that occurred in each area surveyed, and the percentages of sightings of all moth species that occurred in each area. While these are crude measures that do not account for seasonality, they are generally informative, especially for common species. Percentage representation of a site for one species that is much lower that of the site for all moths indicates that the species in question is poorly represented at that site relative to elsewhere on Block Island, and higher representation indicates the reverse. Some species are about equally common at sites across the island, while some have been found exclusively at one surveyed site. Refer to site descriptions for information needed to relate a species’s local distribution on Block Island to its possible host plants and habitat requirements.